A cruel twist of genetic fate brought Alzheimer’s disease to a sprawling Colombian family. But thanks to a second twist, one member of the clan, a woman, managed to evade the symptoms for decades. Her escape may hold the key to halting, or even preventing, Alzheimer’s.
The inherited version of Alzheimer’s disease erodes people’s memories early, starting around age 40. In this family and others, a mutation in a gene called presenilin 1 eventually leaves its carriers profoundly confused and unable to care for themselves. Locals around the Colombian city of Medellín have a name for the condition: la bobera, or “the foolishness.”
The woman in the afflicted family who somehow fended off the disease carried the same mutation that usually guarantees dementia. And her brain was filled with plaques formed by a sticky protein called amyloid. Many scientists view that accumulation as one of the earliest signs of the disease. Yet she stayed sharp until her 70s.
Researchers were stumped, until they discovered that the woman also carried another, extremely rare genetic mutation that seemed to be protecting her from the effects of the first one. This second mutation, in a different Alzheimer’s-related gene called APOE, seemed to slow the disease down by decades, says Joseph Arboleda-Velasquez, a cell biologist at Harvard Medical School.
“There was this idea of inevitability,” he says. But the woman’s circumstances bring “a different perspective” — one in which amyloid buildup no longer guarantees problems. Arboleda-Velasquez and colleagues reported the details of the woman’s exceptional case November 4 in Nature Medicine, omitting the woman’s name and precise age to protect her privacy.
Although the discovery is based on one person, it points to a biological weak spot in the degenerative disease that affects an estimated 5.8 million people in the United States alone. So far, nearly every clinical trial designed to slow or stop the disease has failed. Those heartbreaking disappointments have prompted scientists to expand their search for treatments.
Perhaps this unusually resilient woman in Colombia shows a way to halt the disease, or at least slow it down. “Can we come up with a drug that does this to people who don’t have a mutation?” asks Arboleda-Velasquez. “The potential for that is tremendous.”
Family tree
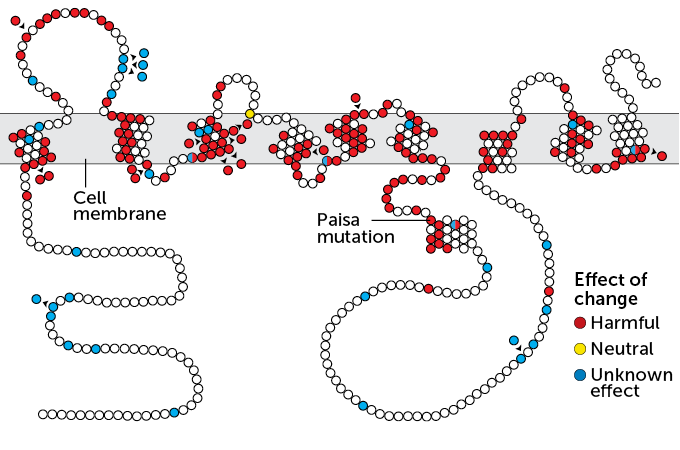
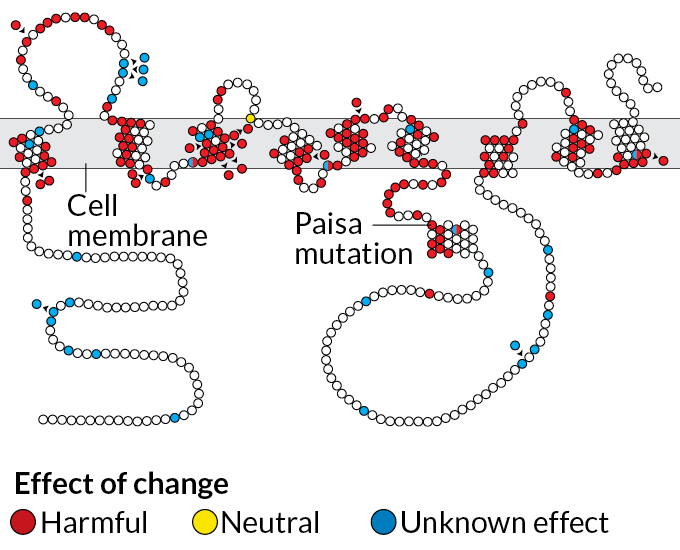
The vast majority of people with Alzheimer’s have a sporadic form of the disease with no clear genetic culprit. These people often reach their 70s or 80s before signs of dementia appear. Mutations that cause trouble much earlier, such as the Paisa mutation found in the Colombian family, are unusual. But despite their different origins and different timelines, these two versions of Alzheimer’s are thought to progress in somewhat similar ways.
Normally, presenilin 1 makes a protein that helps chop up the long, sticky amyloid precursor protein. One of the resulting small bits is called amyloid-beta. Those smaller pieces are harmlessly washed out of the brain. The mutated presenilin 1 gene found in the Colombian family, however, creates a kink in the chopping process that leads to an abundance of a version of amyloid that knits itself into plaques between brain cells.
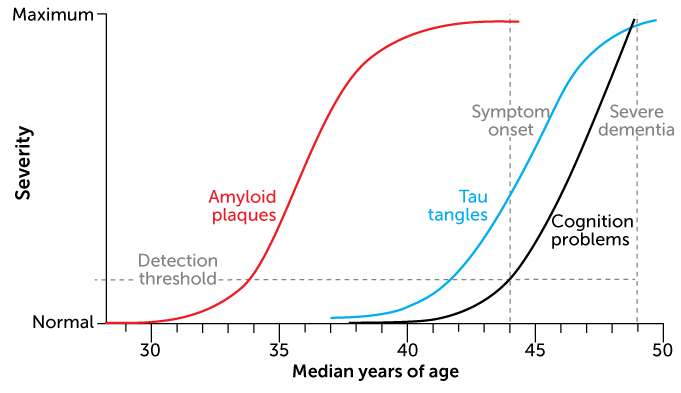
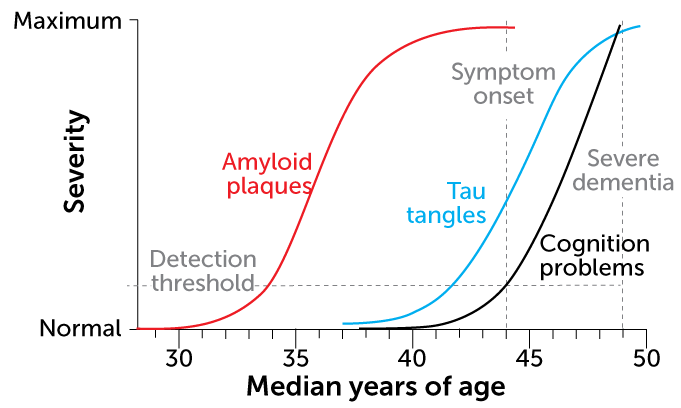
This pileup is already visible in brain scans of people in their 20s who carry the mutation. By their mid-40s, many of these people have trouble remembering; they typically develop full-blown dementia by age 50.
Inheriting just one copy of the mutation is enough to lead to excess amyloid, and ultimately dementia. The mutation’s powerful effect in this family is “one of the strongest arguments for the fact that amyloid plays a critical role” in Alzheimer’s, says immunologist and aging expert Richard J. Hodes, director of the National Institute on Aging in Bethesda, Md. Since taking on the role in 1993, Hodes has helped set the course for U.S.-funded Alzheimer’s research, allocating support for promising projects, including studies happening in Colombia.
The Colombian family, 5,000 members strong, includes an estimated 1,000 or so people who carry the Paisa mutation in the presenilin 1 gene. Their involvement in the research has been invaluable. Access to hundreds of people known to be at high risk for the disease allows scientists to study how Alzheimer’s unfolds, particularly at its earliest stages, and has led to reports of early signs of Alzheimer’s, both in the brain and the blood. Family members have gone to great lengths to help, “walking or taking a bicycle to the nearest bus stop, and then taking a bus to a train, for many hours, to come to the clinic,” Hodes says.
During Hodes’ recent visit to the Medellín area, a resident told him how the disease is just a part of their lives: “If I have the disease, I know that my family, my brother and my sister, will take care of me. And if I don’t, I will take care of them.”
A unique brain
When Colombian researchers learned of the woman who stayed sharp until her 70s, they arranged for her to travel to Boston in the summer of 2016, accompanied by family members and a research assistant. There, neuroimaging researcher Yakeel T. Quiroz and her colleagues used brain scans to measure levels of amyloid and other markers of brain health, including another Alzheimer’s-related protein called tau, which can tangle up inside nerve cells.
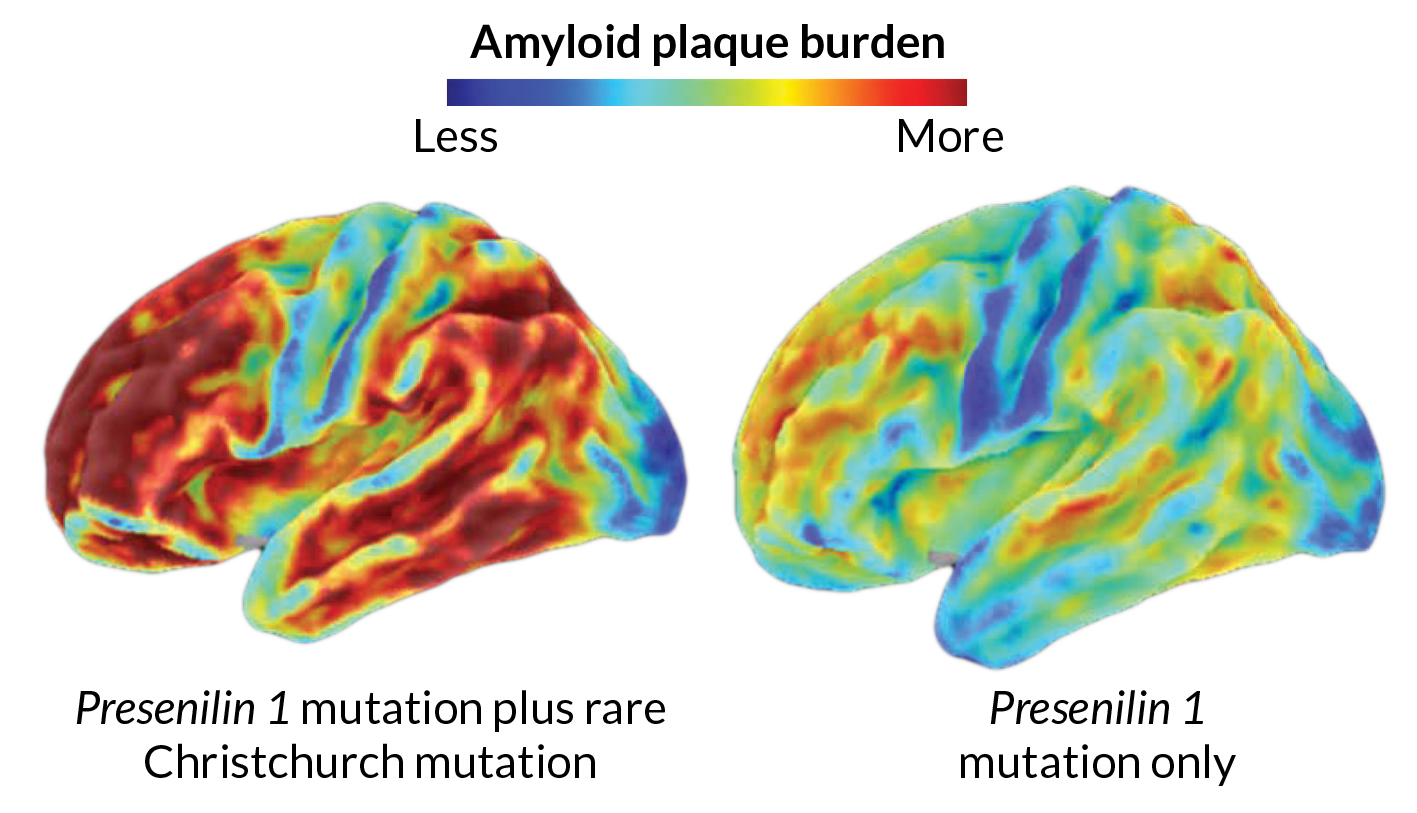
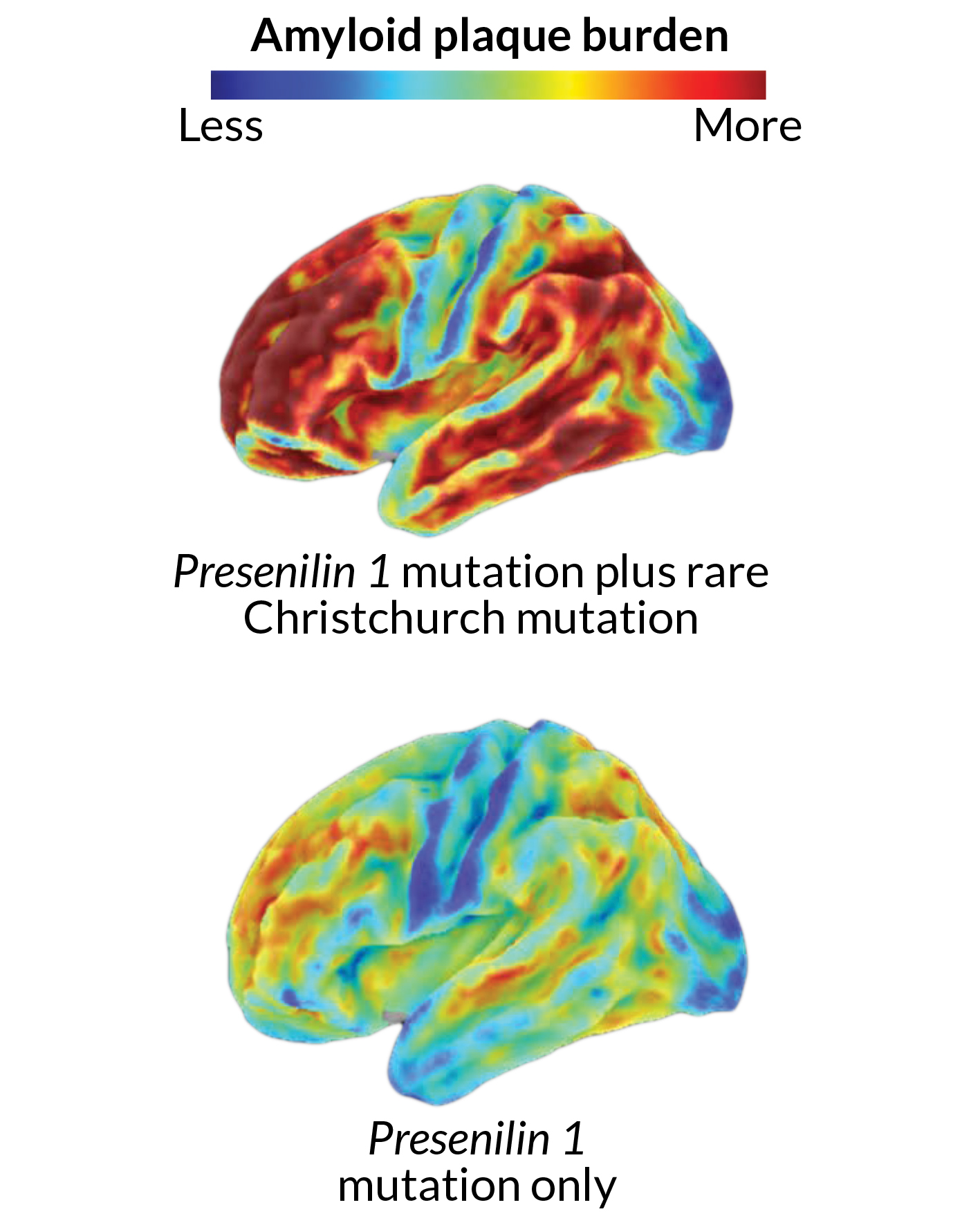
Those scans revealed a brain loaded with amyloid, says Quiroz, of Harvard Medical School. This woman had most likely been accumulating amyloid for decades. On a scale commonly used to quantify amyloid in the brain, she scored 1.96, well above the threshold of 1.2 that signifies extensive amyloid buildup. Her score was, “pretty much the highest that we have seen in anybody we have scanned so far,” Quiroz says.
Genetic analyses revealed that the woman had what’s called the Christchurch mutation in both copies of her APOE gene. Further tests suggested that this mutation, named for the New Zealand city where it was first found, was shielding her from the disease. The fact that the woman had huge amounts of amyloid in her brain, yet didn’t seem impaired until her 70s, is “extremely surprising, interesting, provocative and potentially very, very informative,” Hodes says.
Scientists need to do more work to confirm that the APOE Christchurch mutation protected her brain. Still, the results reveal a simple truth, Hodes says. “Amyloid itself is not necessarily sufficient to cause dementia.”
Studies outside of the Colombian family also make clear that amyloid isn’t the whole story. Other cellular actors contribute to the death of nerve cells and memory loss that Alzheimer’s brings. Nerve cell–clogging tangles of tau and other signs of brain illness are tightly linked to brain decline, research from many studies has shown. That’s reflected in observations from a study of 480 people age 60 and older who live around Rochester, Minn.
These people, none of whom showed signs of dementia, were randomly chosen to be invited into the study, an unbiased selection that offered researchers a glimpse of brain health in the wider population.
To find out which brain changes best predict future memory loss, neuroradiologist Clifford R. Jack Jr. of the Mayo Clinic in Rochester and colleagues tested volunteers’ memory performance while measuring their amyloid levels and other brain signals. Amyloid seemed to be closely involved in memory decline over about five years — but only in the right context, the team reported in June 2019 in JAMA.
Without either of two other troublesome markers — tau tangles or brain shrinkage — amyloid didn’t predict memory loss. In other words, amyloid might be setting up the shot, but then it passes the ball.
Stretching the lag
“Amyloid in the head is the first stage of what will ultimately lead to full-blown Alzheimer’s disease,” Jack says. But there can be a lot of time between that early stage of amyloid accumulation and the development of symptoms.
Among the Colombian family members, that interval lasts around 10 to 15 years. The same is roughly true for people with the sporadic form of Alzheimer’s. But for the woman described in the report in Nature Medicine, that lag seemed twice as long.
“That suggests that at least it’s possible to live with amyloid not just for 15 years, but for many decades,” says Paul Aisen, director of the University of Southern California’s Alzheimer’s Therapeutic Research Institute in San Diego. Living healthy longer: “That’s very exciting.”
The protective effect of the woman’s mutation seems to come from an extremely specific change. In the Christchurch variant, a single spot in the APOE gene is tweaked. The resulting protein has a serine amino acid swapped in for the standard arginine.
The swap prevents the APOE protein from binding to some sugar-dotted proteins called heparan sulfate proteoglycans, or HSPGs, experiments on the isolated proteins revealed. Earlier studies showed that HSPGs may promote amyloid accumulation and nudge nerve cells to slurp up more toxic tau.
But to misbehave, HSPGs might need to partner with the APOE protein. The Christchurch mutation could have protected the woman’s brain by scrambling that nefarious relationship, the researchers suspect. Without that specific connection between APOE and HSPGs, “the disease process gets stalled,” Arboleda-Velasquez says. “This really puts a block on the cascade of events.”
Fleshing out the APOE protein’s normal biological cascade, and how that changes with the Christchurch mutation, is “going to allow for much more finely targeted drug development,” says Aisen, who also works as a consultant for Biogen, a biotechnology company in Cambridge, Mass. The company is developing an amyloid-targeting drug called aducanumab and is expected to apply for approval from the U.S. Food and Drug Administration this year (SN: 1/18/20, p. 8).
As one of the strongest genetic risk factors for dementia, the APOE gene has long been scrutinized as a possible target for Alzheimer’s drugs. People who carry a version of the gene called APOE4 have a higher risk of Alzheimer’s.
The APOE2 version dramatically lowers the risk, Quiroz, Arboleda-Velasquez and colleagues report in preliminary research posted online November 2 at medRxiv.org. APOE3 usually brings an average risk of Alzheimer’s, with the notable exception of the version with the Christchurch mutation carried by the Colombian woman.
Hope for the future
In the general population, old age is the biggest risk factor for Alzheimer’s. As the number of older people balloons, so too will the number of people with dementia. By 2050, an estimated 13.8 million people in the United States will have Alzheimer’s. Worldwide, an estimated 50 million people have dementia; Alzheimer’s accounts for the bulk of those cases.
The family in Colombia continues to help. A clinical trial testing a drug that is designed to lower amyloid is under way in Colombia. People who have the Paisa mutation but have not shown Alzheimer’s symptoms, as well as people without the mutation, are receiving the drug. The drug, crenezumab, is an antibody that’s thought to mark amyloid for destruction by immune cells. It’s being developed by Roche/Genentech.
Quiroz and her colleagues also plan to follow the Colombian woman and other members of the family over time, as part of a research exchange between Fundación Universidad de Antioquia in Medellín, which has led the studies on this family, and Massachusetts General Hospital in Boston.
Each month, the project, called COLBOS, for Colombia-Boston, flies a new group of about five adult participants to Boston for extensive evaluation, including thinking and memory tests, brain scans and measurements of smelling ability, fitness and music perception. Participants being studied in Colombia are as young as 9 years old.
The project may yield insights about how Alzheimer’s takes hold early on. But in a way, the initial trigger might not even matter. It could be that the cause — or more likely, causes — of Alzheimer’s might ultimately be poor targets for drugs, Arboleda-Velasquez says.
People with loved ones suffering from Alzheimer’s, including the Colombian family, don’t necessarily care what causes the disease, Quiroz says. “They are more interested in seeing if there is anything that can help them to get better. That’s what the patients and families are waiting for.”